Overview
Biomedical sensors are fundamental tools in biomedical science and technology. Accurate biomedical research conclusions depend on correct measurements from these sensors. Sensor development integrates multiple scientific and engineering disciplines.
Basic Sensor Model
In a typical sensor model, a sensitive membrane serves as the interface between the sensor and the measured object. Adding a purpose-designed membrane to a conventional sensor can produce chemical sensors or biosensors; the key distinction is whether the membrane has biological activity. A biologically active membrane defines a biosensor. Two interfaces may exist: between the measured medium and the sensitive membrane, and between the sensitive membrane and the sensor itself. Complex physical, chemical, or biological processes occur at these interfaces.
Requirements for Medical Sensors
- High safety, especially for sensors and transducers used on or in the human body; high sensitivity and high signal-to-noise ratio (high selectivity).
- Measures to ensure electrical safety include galvanic isolation and floating techniques.
- Measures to ensure chemical safety require non-toxicity and no short- or long-term carcinogenic effects.
- Measures to ensure biological safety require no DNA or RNA mutagenicity.
- Approaches to ensure high selectivity include resonance effects, filtering techniques, adaptive methods, molecular recognition, and ion-recognition technologies.
- Approaches to increase sensitivity include physical, chemical, and biochemical amplification techniques.
Main Medical Uses
- Detecting biological information: for example, measuring intracardiac pressure before cardiac surgery; basic research in cardiovascular disease may require measurement of blood viscosity and lipid content.
- Clinical monitoring: continuous monitoring of physiological parameters such as body temperature, pulse, blood pressure, respiration, and electrocardiography before, during, and after surgery.
- Control: using measured physiological parameters to control physiological functions, for example electronic prostheses.
Quantities Measured in Medicine
Biomedical sensors are used to measure a range of physical, chemical, and electrical quantities relevant to human physiology and clinical practice.
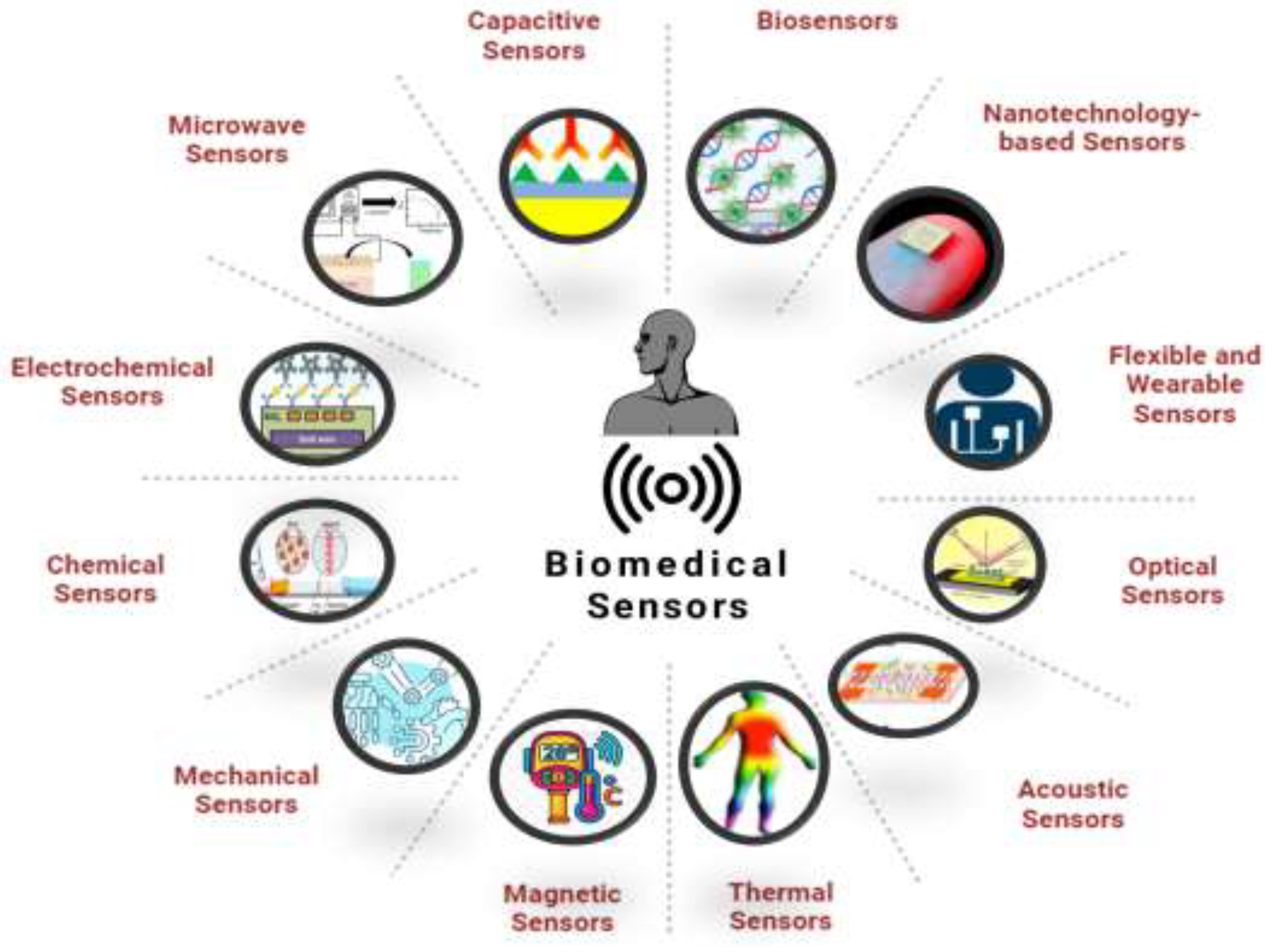
Classification of Biomedical Sensors
By application form: implantable sensors, temporarily implanted cavity or incision sensors, external sensors, and sensors for external devices.
By working principle: physical sensors (displacement, force, temperature, humidity, etc.), chemical sensors (various chemical species), biosensors (enzymes, immunoassays, microorganisms, DNA, etc.), and bioelectrical electrodes (ECG, EEG, EMG, neuronal discharges, etc.).
Physical Sensors
Physical sensors convert physical quantities into electrical signals readable by computers. Examples used in biomedical applications include:
- Force sensors for weight measurement.
- Piezoelectric film sensors for heart rate and respiration pattern monitoring.
- Thermopile sensors for body temperature measurement.
- Pulse oximetry sensors for blood oxygen content.
- CO2 sensors for measuring metabolic activity.
- Flow sensors to assist mechanical ventilation.
- Force sensors to measure remaining oxygen in oxygen cylinders.
Chemical Sensors
Chemical sensors convert chemical composition or concentration into an electrical quantity with a defined relationship to the analyte. Many chemical sensors rely on functional membranes that selectively interact with target chemical species, with an electrochemical transducer converting the selected species into an electrical signal.
Classification often depends on the response mechanism, membrane composition, or membrane structure, for example: ion-selective electrode transducers, gas-sensitive electrodes, humidity-sensitive electrodes, coated-wire electrodes, polymer-matrix electrodes, ion-sensitive field-effect transistors (ISFETs), ion-selective microelectrodes, and ion-selective thin-film transducers.
Chemical transducers used in biomedical applications can measure species such as K+, Na+, Ca2+, Cl-, O2, CO2, NH3, H+, Li+, and others.
Biosensors
Biosensors use biologically active substances for selective recognition and measurement. They typically comprise two main components: a molecular recognition element that selectively binds the analyte, and a transducer that converts the biochemical interaction into an electrical or optical signal.
The earliest biosensor was the enzyme electrode, as proposed by Clark and Lyons. In the mid-1970s, limitations such as short enzyme lifetimes and high cost of purified enzymes led to derived types of enzyme electrodes, including microbial electrodes, organelle electrodes, tissue-based electrodes, and immunoelectrodes, expanding biosensor categories.
From the 1980s onward, with advances in ion-sensitive field-effect transistors, enzyme FETs were developed that could, for example, determine penicillin.
Components and Basic Principles
- Molecular recognition element.
- Transducer.
Transducer types include electrochemical electrodes, semiconductors, thermistors, surface plasmon, and piezoelectric crystals.
Classification by Recognition Element
Biosensors can be classified according to the recognition element employed, such as enzymes, microbes, cells, antibodies/antigens, DNA, and others.
Enzyme Sensors
Enzyme catalysis accelerates substrate decomposition under defined conditions. Enzyme sensors consist of immobilized enzyme on a base electrode. Design focuses on detecting substances produced or consumed by the catalytic reaction. For example, if an enzyme-catalyzed reaction consumes O2, an O2 electrode or an H2O2 electrode may be used; if the reaction produces acid, a pH electrode may be employed.
Signal transduction methods include potentiometry and amperometry.
Glucose Sensors
Operating Principles
Glucose sensors typically use glucose oxidase (GOD) and operate by one of three methods: measuring O2 consumption, measuring H2O2 production, or measuring pH change resulting from gluconic acid formation.
Reaction: glucose + H2O + O2 -> gluconic acid + H2O2
Measuring O2 Consumption
An oxygen electrode can be constructed with a Pb anode and a Pt cathode immersed in alkaline solution, with the cathode surface covered by an oxygen-permeable membrane (e.g., Teflon, thickness ~10 μm). When a polarization voltage (e.g., 0.7 V) is applied, O2 diffusing through the membrane is reduced at the Pt cathode. The resulting current is proportional to dissolved O2 concentration:
O2 + 2 H2O + 4 e- -> 4 OH-
Measuring H2O2 Production
Glucose oxidase catalyzes glucose oxidation producing H2O2. H2O2 diffuses through a selective membrane and is oxidized at a Pt electrode, generating anodic current proportional to glucose concentration. At approximately +0.6 V applied to the Pt electrode, the reaction is:
H2O2 -> O2 + 2 H+ + 2 e-
Microbial Sensors
Microbial sensors can be aerobic or anaerobic. When a sensor is placed in a solution containing organic compounds, the organics diffuse into the microbial film and are metabolized.
Aerobic Microbial Sensors
Microbial respiration can be monitored using oxygen or carbon dioxide electrodes.
Anaerobic Microbial Sensors
Anaerobic sensors measure microbial metabolic products, which can be detected by ion-selective electrodes.
Example: Formate Sensor (Anaerobic)
Butyric acid-producing Clostridium species that generate H2 can be immobilized in a low-temperature gelatin film and placed on a fuel-cell Pt electrode. When the sensor is immersed in a solution containing formate, formate diffuses through a PTFE membrane and is metabolized by the bacteria to produce H2. H2 diffuses back through the PTFE layer to the Pt electrode, where redox reactions generate a current proportional to H2 production, and thus proportional to the formate concentration in the fermentation solution.
Immunosensors
Immunosensors are based on immune reactions. Immobilized antibodies or antigens specifically bind the corresponding antigen or antibody, causing changes in the potential of the biological sensing membrane. For example, when an antigen is immobilized on an acetylcellulose membrane, the protein's bipolar electrolyte properties result in surface charge that varies with pH; membrane potential changes can be used to quantify bound antibody.
Trends and Developments
Medical sensor technology has evolved from large, low-performance devices to approaches emphasizing intelligence, miniaturization, multi-parameter sensing, remote operation, and noninvasive detection. Emerging sensor types such as DNA sensors and fiber-optic sensors are also under development. Advances in sensor technology are driving further progress in clinical practice and biomedical research.
 ALLPCB
ALLPCB