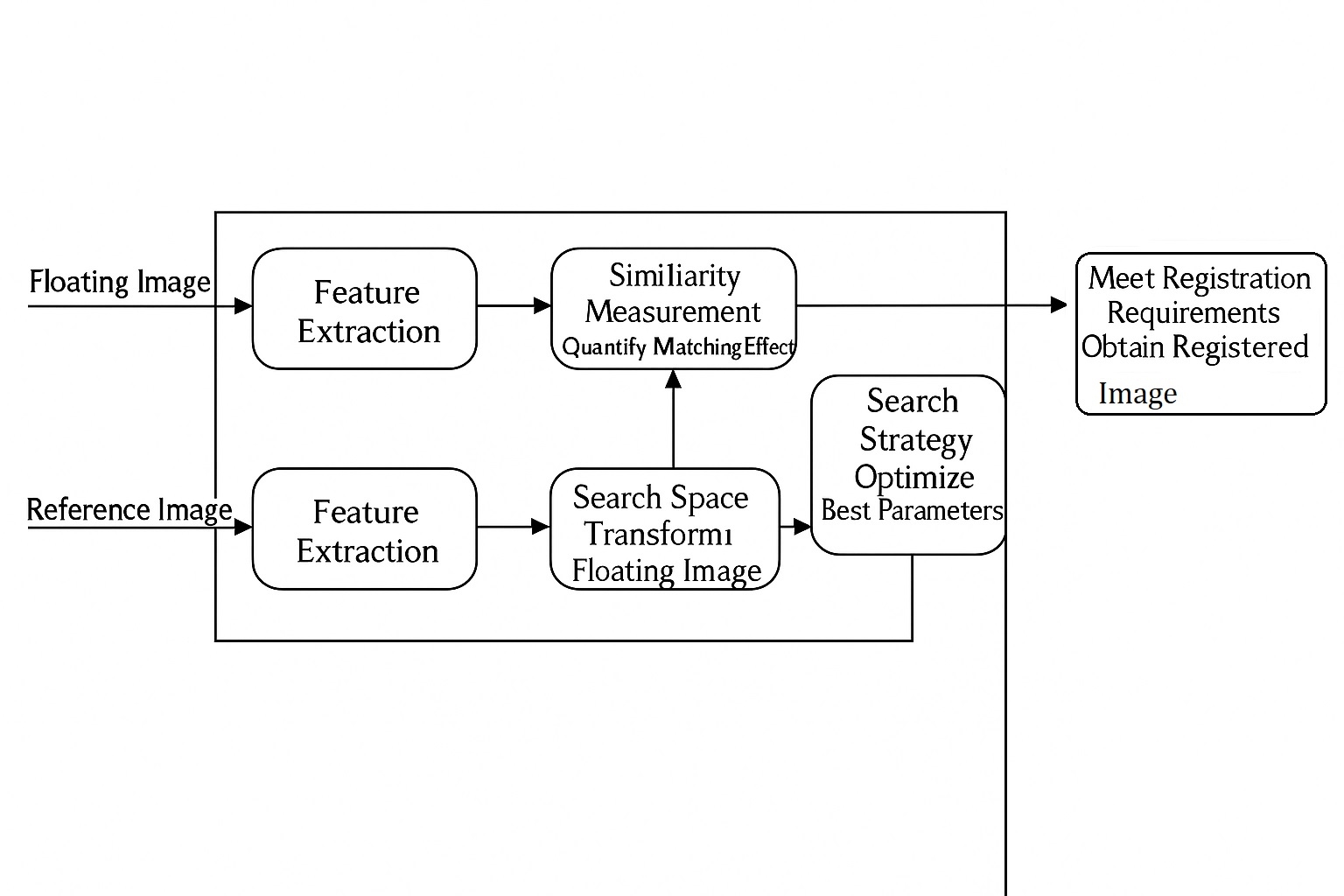
Conventional intravenous (IV) needles, due to their rigid nature, can cause tissue damage and increase the risk of pathogen transmission. To address these issues, a team from the Korea Advanced Institute of Science and Technology, led by Jae-Woong Jeong and Won-Il Jeong, developed an IV needle with temperature-dependent stiffness and shape. This needle is rigid enough for soft tissue insertion but softens irreversibly after insertion to conform to blood vessels, reducing damage during removal. Additionally, an integrated thin-film temperature sensor enables core body temperature monitoring in mice and detects fluid leakage in pig tissue ex vivo. The research was published in Nature Biomedical Engineering under the title ¡°A temperature-responsive intravenous needle that irreversibly softens on insertion.¡±
Needle Design and Functionality
The temperature-sensitive IV needle consists of two main components: a rectangular needle frame and a soft polymer encapsulation. The frame, made of gallium, features two U-shaped groove structures with upper and sidewall thicknesses of 0.20 mm and 0.10 mm, respectively. The outer U-shaped frame encloses the inner platform, forming a hollow rectangular structure sealed with silicone for high tear strength. Gallium was chosen for its phase-transition properties, low glass-transition temperature, and biocompatibility, making it ideal for variable-stiffness medical needles. A thin-film temperature sensor can be embedded to monitor body temperature in real time.
At room temperature, the needle is rigid and straight, similar to conventional medical needles. When exposed to temperatures of approximately 30¡ãC or higher, the gallium frame liquefies, rendering the needle soft and flexible. This allows reliable fluid delivery even in a highly deformed state. After use, the needle remains fully softened, preventing reuse. This design enhances patient comfort, improves compliance, and reduces the risk of accidental needle injuries or pathogen transmission through bloodborne routes.
Thermal and Mechanical Properties
Understanding the thermal behavior of the temperature-sensitive IV needle is critical for characterizing its stiffness transition. When exposed to a simulated biological environment at 37¡ãC, the needle transitions to a soft state in approximately 60 seconds, with negligible volume change during the phase shift. The transition time can be reduced by decreasing the thickness of the gallium and polymer sealant. In rigid mode, the needle exhibits high bending stiffness for soft tissue insertion. In its soft state, stiffness decreases significantly, enabling dynamic deformation and minimizing mechanical damage to vessel walls.
To evaluate tissue penetration, the needle was tested on an artificial tissue model. Results showed that its compressive strength can overcome the stress required for skin puncture, with an equivalent force of 1 N. The choice of encapsulation material is critical for safe and rigid insertion.
Integrated Temperature Sensing
For enhanced patient care, a thin-film temperature sensor with a serpentine gold film structure was integrated near the needle tip to monitor core body temperature or detect therapeutic drug leakage. The sensor¡¯s ultra-thin and flexible design ensures seamless interfacing. Testing demonstrated that the needle can monitor body temperature in real time during infusion and instantly detect drug leakage.
Applications and Implications
The temperature-sensitive IV needle offers biomechanical compatibility and single-use safety. Its applications extend to various clinical settings, and it supports global health initiatives, such as those by the World Health Organization, to prevent needle reuse and unsafe injection practices.
 ALLPCB
ALLPCB