EMC (Electromagnetic Compatibility) and MEA (Medical Electrical Equipment) are two distinct concepts in the electronics and electrical engineering fields, each with different definitions and applications.
1. EMC (Electromagnetic Compatibility)
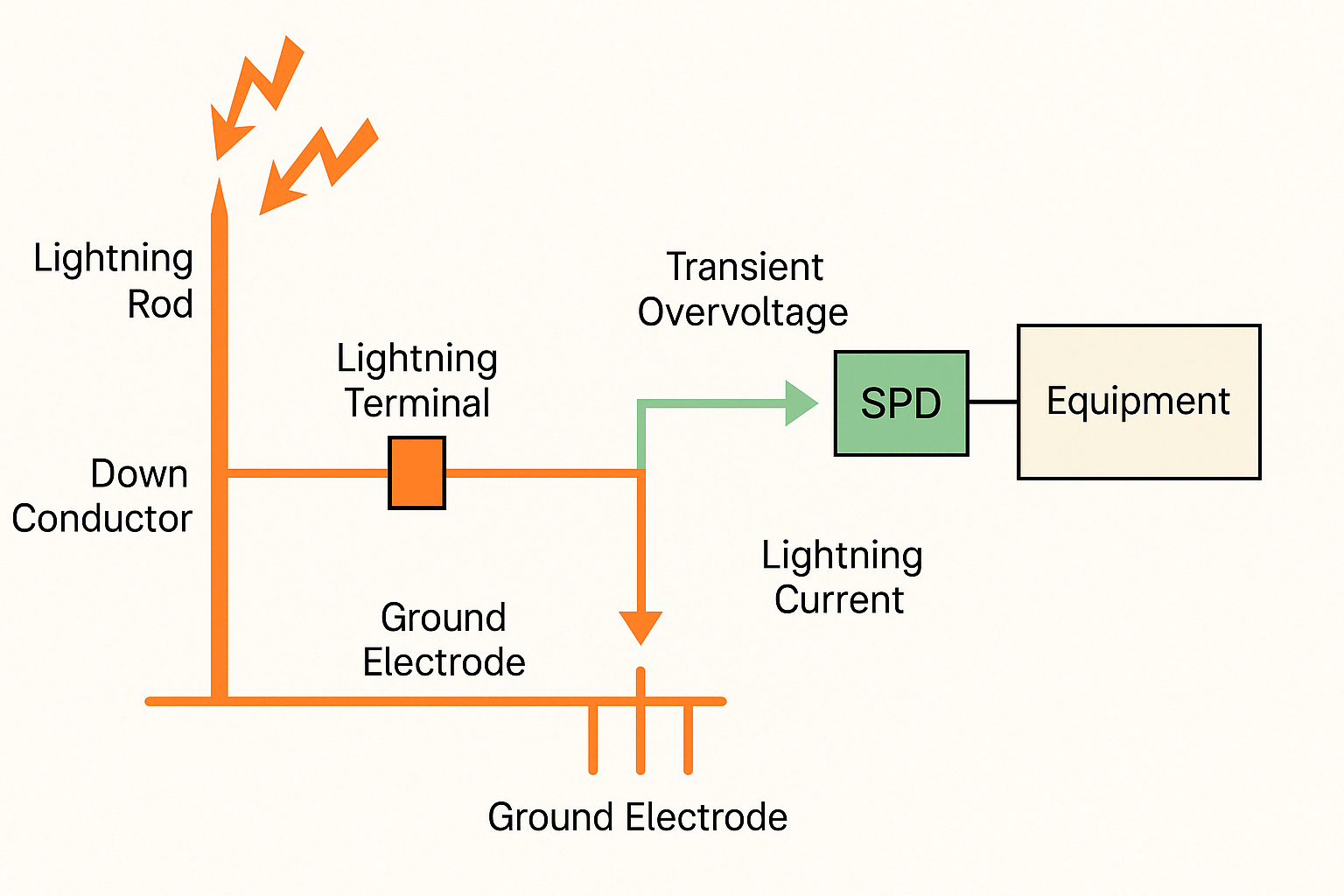
EMC is the ability of an electronic or electrical device to function at an appropriate performance level within its specific electromagnetic environment without introducing intolerable electromagnetic disturbances to other equipment or the environment. In other words, EMC ensures that devices can operate in proximity to one another without causing mutual interference or being affected by external electromagnetic disturbances. The field of EMC involves compatibility testing, regulations, and standards to ensure a device's electromagnetic performance.
Three Components of Electromagnetic Interference (EMI)
- Interference source
- Coupling path
- Susceptible device (receiver)
2. MEA (Medical Electrical Equipment)
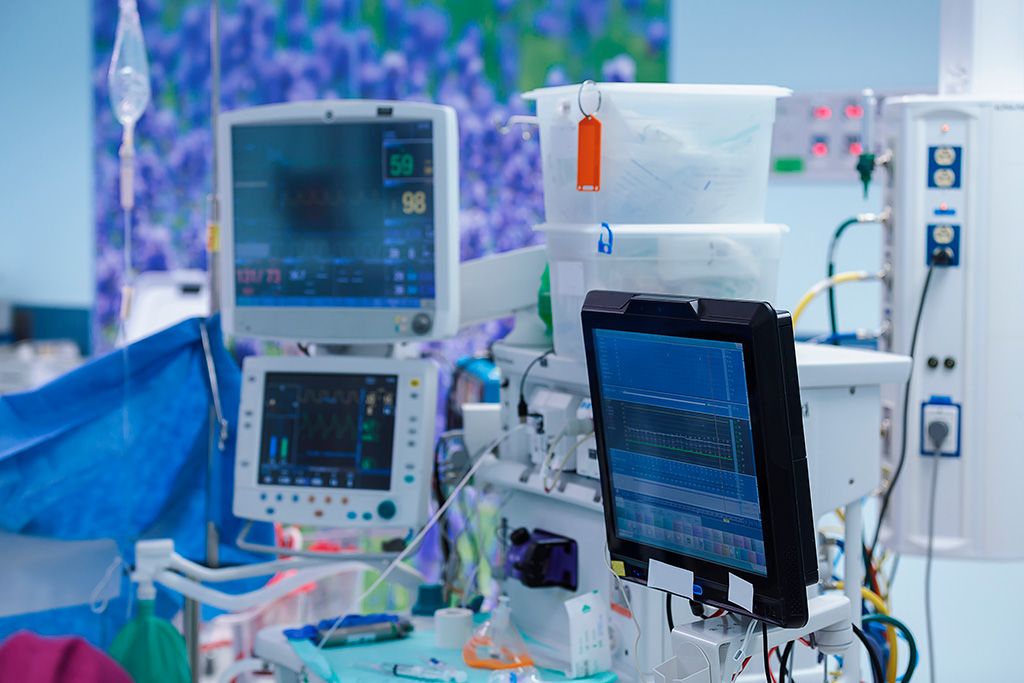
MEA refers to electrical equipment intended for medical purposes. It encompasses a wide range of devices used for diagnosis, treatment, monitoring, illumination, and other medical applications, such as ECG machines, X-ray equipment, and medical ultrasound devices. MEA must comply with specific standards and requirements during design and manufacturing to ensure its safety and effectiveness.
MEA plays a crucial role in the medical device sector and is widely used in hospitals, clinics, laboratories, and other healthcare facilities.
Below is a detailed overview of MEA:
- Types and Scope of Application: MEA includes various types of devices for processing, monitoring, and influencing human physiological processes. These can be categorized as medical imaging equipment (e.g., X-ray machines, CT scanners, MRI machines), surgical equipment (e.g., electrosurgical units, electric operating tables), life support equipment (e.g., pacemakers, ventilators), monitoring equipment (e.g., ECG machines, blood pressure monitors), and laboratory equipment (e.g., centrifuges, microscopes). Each device has a specific function and purpose.
- Safety and Regulatory Standards: The safety of MEA is paramount. Medical electrical equipment must adhere to specific safety standards and regulations to prevent harm to patients and healthcare personnel. For example, the internationally adopted IEC 60601 series of standards specifies the safety and performance requirements for medical electrical equipment.
- Design and Manufacturing: The design and manufacturing of MEA are based on the special requirements of medical applications. This equipment must account for human physiological characteristics, medical procedures, and operational needs. The design must ensure patient safety and be easy for medical staff to operate and maintain. Strict quality control is required during manufacturing to guarantee the device's reliability and stability.
- Technological Innovation: The demand for technological innovation in MEA is constantly growing. The introduction of new technologies, such as remote monitoring, artificial intelligence, and biosensors, is transforming medical devices. These innovations not only improve patient treatment outcomes and experiences but also enhance the efficiency and precision of medical procedures.
- Clinical Validation and Use: MEA must undergo clinical validation and evaluation to confirm its safety and efficacy. This equipment may need to interoperate with other medical devices, systems, and procedures and require close coordination with healthcare professionals. Therefore, validating and assessing MEA in a real-world clinical environment is essential.
Key Differences
- Focus: EMC focuses on electromagnetic compatibility issues between devices, i.e., how they coordinate their operation within an electromagnetic environment. It emphasizes communication and mutual interference problems. In contrast, MEA concentrates on the safety and performance of the medical electrical equipment itself to ensure its effectiveness and reliability in medical applications.
- Concerns: EMC is primarily concerned with issues like electromagnetic radiation and susceptibility, focusing on the electromagnetic emissions a device produces and its interference with other equipment. MEA, however, is more concerned with the device's safety, reliability, and effectiveness, along with related electrical safety standards and regulations, to ensure it is harmless and compliant in a medical setting.
- Scope: EMC is a broad concept applicable to electronic and electrical equipment across various fields. MEA is a specific concept confined to the medical sector, applying only to medical electrical equipment.
In summary, while there are some overlapping aspects, EMC addresses the electromagnetic compatibility between devices, whereas MEA focuses on the safety and performance of medical electrical equipment. They have distinct differences in their definitions and applications.
 ALLPCB
ALLPCB