Diabetes is a disease characterized by elevated blood glucose concentration. The World Health Organization reports that diabetes affects billions of people worldwide and, without timely treatment, can lead to severe complications such as renal failure, blindness, and stroke. A cure for diabetes remains elusive, and continuous monitoring of a patient’s glucose concentration is an important means of tracking disease progression.
Recent review
According to Mems Consulting, researchers at Hangzhou Dianzi University published a review in the Journal of Biomedical Engineering titled "Progress in the Application of Wearable Noninvasive Glucose Sensors in Diabetes Management." The article summarizes recent advances in wearable noninvasive glucose sensors and their use in diabetes management, outlines the advantages of wearable sensors and the challenges encountered during research and development, and discusses commercialization trends and market potential.
Types of wearable noninvasive glucose sensors
Based on the type of biofluid sampled, wearable noninvasive glucose sensors can be classified into four main categories: sensors for interstitial fluid (ISF), sweat, tears, and saliva.
1) Interstitial fluid glucose sensors
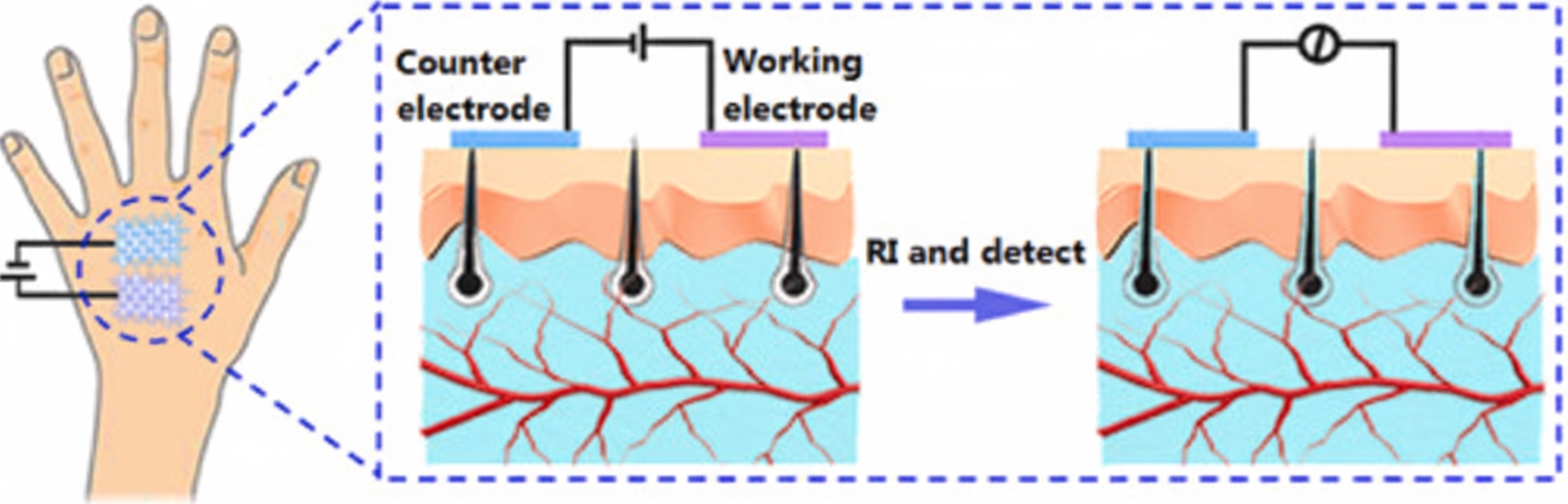
Apart from blood, interstitial fluid is the best source for obtaining glucose concentration in terms of both accuracy and dynamics. Interstitial fluid surrounds skin cells and supplies nutrients via diffusion from capillary endothelium, which creates a reliable correlation between blood and interstitial glucose concentrations. Reverse iontophoresis (RI) has been widely studied as a method to extract interstitial glucose, but current challenges include complex device structures and instability.
Yao et al. demonstrated a simple dual-electrode noninvasive glucose sensor that uses graphene/carbon nanotube/glucose oxidase composite fabric as the working electrode and graphene/carbon nanotube/silver/silver chloride composite fabric as the counter electrode. Interstitial fluid was extracted by reverse iontophoresis: a current was first applied between the electrodes, and glucose concentration in the extracted interstitial fluid was then detected amperometrically. The sensor’s feasibility was validated on porcine skin, nude mice, and humans, and calculated blood glucose concentrations were highly consistent with commercial blood glucometers.
2) Sweat glucose sensors
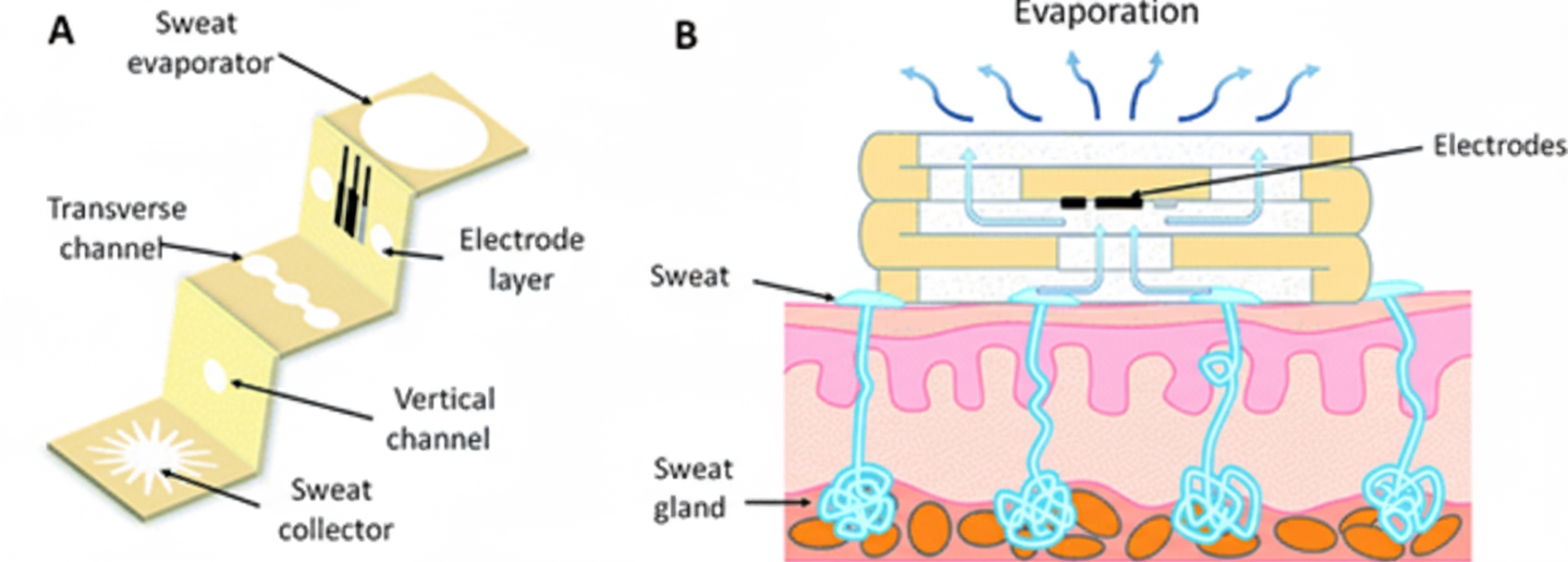
Sweat is the most accessible biofluid for chemical sensing applications. It contains many metabolites, electrolytes, trace elements, and small amounts of macromolecules. Sweat analysis can provide noninvasive monitoring of physiological health and support disease diagnosis and management. Because people with diabetes have higher glucose concentrations in the body, more glucose may be excreted in sweat, so sweat analysis can be used to monitor systemic glucose. However, accurate measurement of sweat glucose faces significant challenges such as temperature and pH variations, contamination from other biomarkers or old sweat, small sampling volumes, and uncontrolled evaporation. Sweat glucose concentrations are also low (typically 0.02 mM to 0.6 mM), requiring highly sensitive sensors.
To improve detection sensitivity, Yu et al. used gold nanoneedle structures as a signal amplification strategy. They deposited gold nanoneedles on the working electrode by electrochemical deposition to create a sensor for real-time monitoring of glucose and lactate in sweat. Enzyme immobilization was performed on the electrode surface using a crosslinker, polyethylene glycol polyglycerol ether, which preserved enzyme activity more effectively than glutaraldehyde. The increased specific surface area from the deposited structures allowed more enzyme to be immobilized, improving sensitivity.
To address performance degradation caused by sweat accumulation, Cao et al. proposed a three-dimensional paper-based microfluidic electrochemical integrated device (3D-PMED) for real-time monitoring of sweat metabolites. The device integrates a screen-printed glucose sensor on a PET substrate with a fabricated 3D-PMED. Red ink was used to simulate sweat flow within the 3D-PMED, demonstrating its ability to collect, analyze, and evaporate sweat. Human trials validated the practicality of the 3D sweat monitoring device.
3) Tear glucose sensors
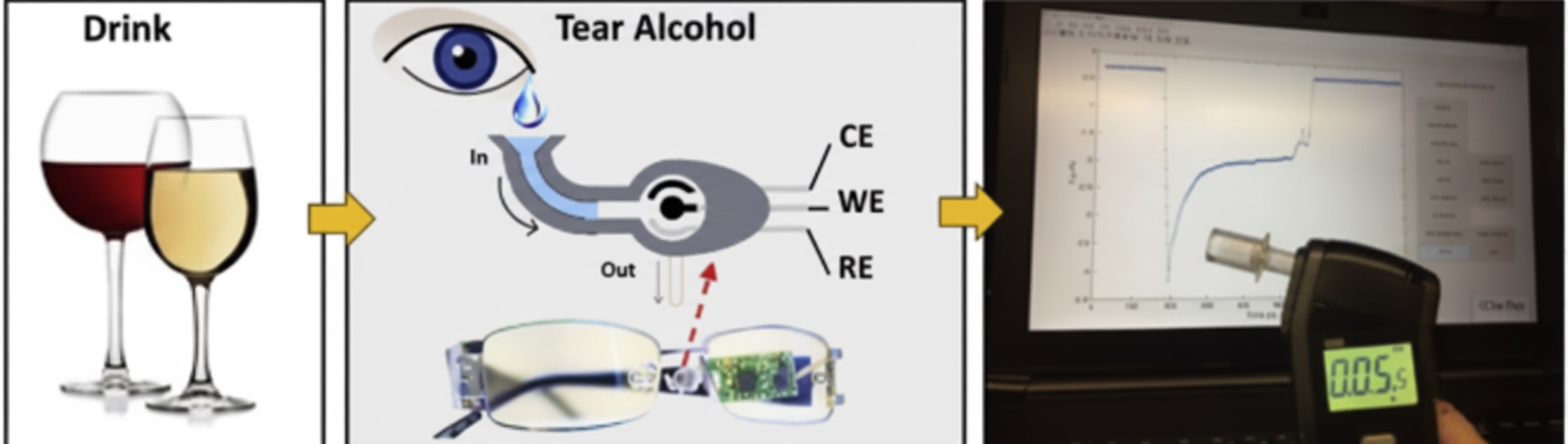
Biomarkers in tears can diffuse directly from blood and show a strong correlation between tear and blood glucose concentrations. These properties make tears an effective biofluid for health monitoring. Studies indicate that tear glucose correlates with blood glucose in both healthy subjects and people with diabetes: average tear glucose in diabetes patients has been reported at about 0.92 mM, compared with about 0.2 mM in healthy subjects. As with sweat, tear glucose responds to changes in blood glucose with a lag; March et al. reported that tear glucose changes lagged blood glucose by about 5 minutes after oral glucose intake.
Sempionatto et al. reported a wearable tear bioelectronic platform that integrates a microfluidic electrochemical detector into the nose-pad of eyeglasses. The tear sensing platform is positioned outside the eye region and incorporates a wireless electronics module into the eyeglass frame, creating a fully portable and convenient sensor. The device can monitor blood alcohol concentration via tears, as well as glucose and vitamin levels.
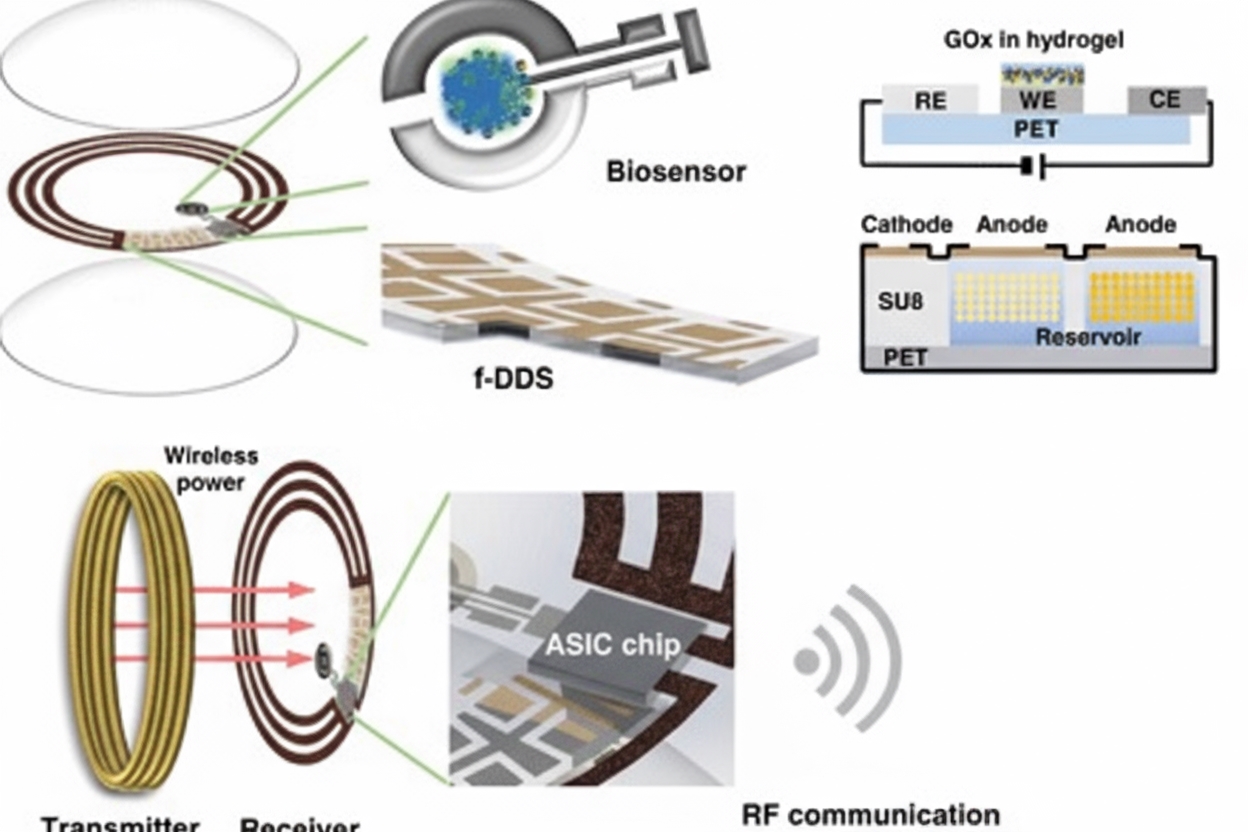
Keum et al. developed a smart contact lens capable of both glucose monitoring and therapy for diabetic retinopathy. The lens is constructed on a biocompatible polymer and integrates ultrathin, flexible circuits and a microcontroller chip. The system performs real-time glucose monitoring, on-demand drug delivery, wireless power management, and data communication. In a rabbit model of diabetes, tear glucose measurements were validated against conventional blood glucose tests.
4) Saliva glucose sensors
Research on saliva as a diagnostic fluid has advanced rapidly. Many salivary biomarkers enter saliva from blood via extracellular or paracellular pathways, reflecting physiological status and enabling noninvasive glucose analysis. Saliva is easy to collect and has been widely used in biosensors and portable diagnostic platforms. Saliva diagnostics, particularly metabolomics studies of numerous low-molecular-weight endogenous metabolites, has become an important tool for detecting many diseases, including diabetes.
Carmona et al. developed a pacifier-based wearable platform for monitoring glucose in neonatal saliva. When an infant sucks the pacifier, mouth motion pumps saliva effectively and directs it unidirectionally toward an external electrochemical chamber. The device was evaluated in adult patients with type 1 diabetes and showed good correlation with blood glucose levels. The device’s detection limit was 0.04 mM with a sensitivity of (0.69 ± 0.04) nA/mM/cm2.

Summary and outlook
Given the current diabetes burden in China, traditional monitoring, treatment, and care methods are insufficient, and new medical approaches are needed to improve diabetes management. In recent years, flexible electronics have advanced rapidly, and by integrating sensor technology, wearable flexible electronic devices have begun moving from lab research toward clinical application. As glucose sensors improve in accuracy and selectivity, noninvasive monitoring of blood glucose trends is likely to become more feasible. Despite the promise, these sensors face several challenges beyond ensuring necessary accuracy and selectivity.
Key challenges
- Safety
Wearable glucose sensors are applied in medical contexts, so safety is paramount. Safety considerations include electrical safety, material biocompatibility, structural integrity, biological side effects, and data privacy. The first four aspects ensure the user is not harmed during wear and measurement and support sensor stability and data reliability. Because sensor data involve sensitive personal information, robust privacy protection is also required.
- Power supply
Power is another challenge. Sensor power consumption mainly comes from three areas: 1) powering the biosensing elements; 2) data processing; and 3) wireless communication. Selecting an appropriate energy source is important: batteries that are too large reduce wearing comfort, while batteries that are too small limit operating life. Balancing comfort and battery life is a major design challenge.
- Data reliability
Sensor measurements will be used to guide patient treatment, so unreliable data can worsen a patient's condition. Compared with invasive blood glucose testing, measuring glucose in interstitial fluid, sweat, tears, or saliva is more susceptible to external interference, and glucose concentrations in these fluids may differ in their correlation with blood glucose. Sensors therefore require better selectivity and higher sensitivity, along with strategies to mitigate interference. Improving selectivity and sensitivity is key to addressing this challenge.
Wearable noninvasive glucose sensors still need to overcome limitations related to time, spatial resolution, and geographic factors to meet the demands of personalized care. As a promising detection technology, China needs to strengthen research and development efforts to further advance these sensors so they can more effectively support diabetes management.
 ALLPCB
ALLPCB