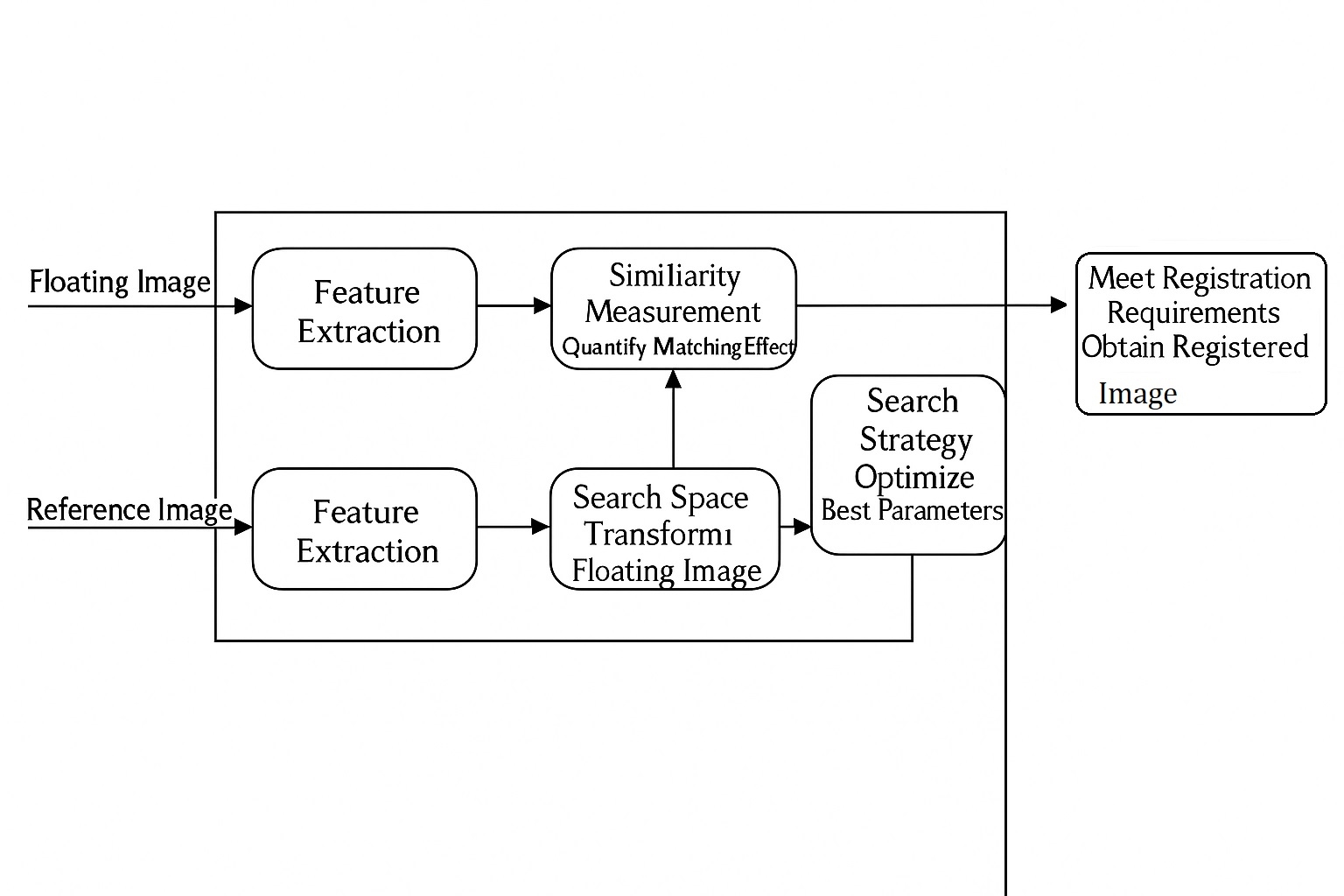
Overview
Brain-computer interface (BCI) systems can establish a direct communication bridge between living nervous tissue and external devices. Noninvasive BCIs typically place sensors on the skull to detect the electrical activity produced by millions of neurons simultaneously. Advances in electronics, materials science, and artificial intelligence have driven neural devices toward miniaturization, improved intelligence, and reduced invasiveness and immune response, expanding their potential applications, including restoring communication for people with aphasia.
Recent review and clinical milestones
The Chinese Academy of Engineering journal Engineering published a 2022 review titled "Using Brain-Computer Interfaces to Help People With Aphasia," summarizing major research directions and findings in the field. Early BCI work focused on restoring motor function for paralyzed patients. As the field has progressed, applications have expanded to restoring vision and hearing, treating psychiatric and neurological disorders, and enabling communication for people with paralysis. Practical deployment also requires consideration of market and cost factors.
In July 2021, The New England Journal of Medicine reported that a neural implant enabled a patient who had been paralyzed for 18 years to produce an intelligible spoken sentence for the first time: "My family is outside." The patient, referred to as Pancho (pseudonym), was 20 when he suffered a severe car accident in 2003. While this result involved a single patient, it marked a milestone in BCI engineering. Researchers at the University of California, San Francisco (UCSF) developed algorithms that controlled an electronic array over language regions of Pancho's brain and translated his neural activity into speech. Each year, thousands of people lose expressive ability due to stroke, disease, or injury, and researchers hope these technologies can eventually restore communication for such populations.
Implantable BCIs
Implantable BCIs involve surgical placement of devices into or on the brain. These devices are often arrays of dozens to hundreds of small metal electrodes targeted at specific neurons, capable of both recording and stimulating neural activity. Although implanted devices can directly access brain signals, they have clear limitations. All surgery carries risks, and intracortical implants act as foreign bodies within cortical tissue. Over time, glial scar formation can envelop electrodes, reducing their ability to stimulate or record reliably.
Noninvasive BCIs
Noninvasive BCIs place sensors on the scalp to detect the aggregate electrical fields generated by large populations of neurons. These sensors can be applied or removed without surgery, but the recorded signals are typically lower in spatial resolution and signal-to-noise ratio compared with invasive approaches. As one researcher described it, the difference is like listening to two people speak from beside them versus from another room.
Clinical applications beyond motor restoration
While early BCI development prioritized restoring movement in paralyzed patients, applications now include sensory restoration, psychiatric and neurological treatment, and communication aids. In 2005, after extensive animal research, a multi-institution team implanted an early BCI in a tetraplegic patient to control a robotic hand. Since then, BCIs have advanced to provide full three-dimensional prosthetic control and haptic feedback.
BCIs are also used to treat a range of psychiatric and neurological disorders. In 1997 the U.S. Food and Drug Administration approved the first deep brain stimulation (DBS) system for essential tremor. DBS systems, essentially long electrodes with multiple contacts controlled by an implanted pulse generator, were later approved for Parkinson's disease and other movement disorders, and more recently for epilepsy. DBS has shown substantial improvements in motor scores for some patients, enabling return to work in many cases.
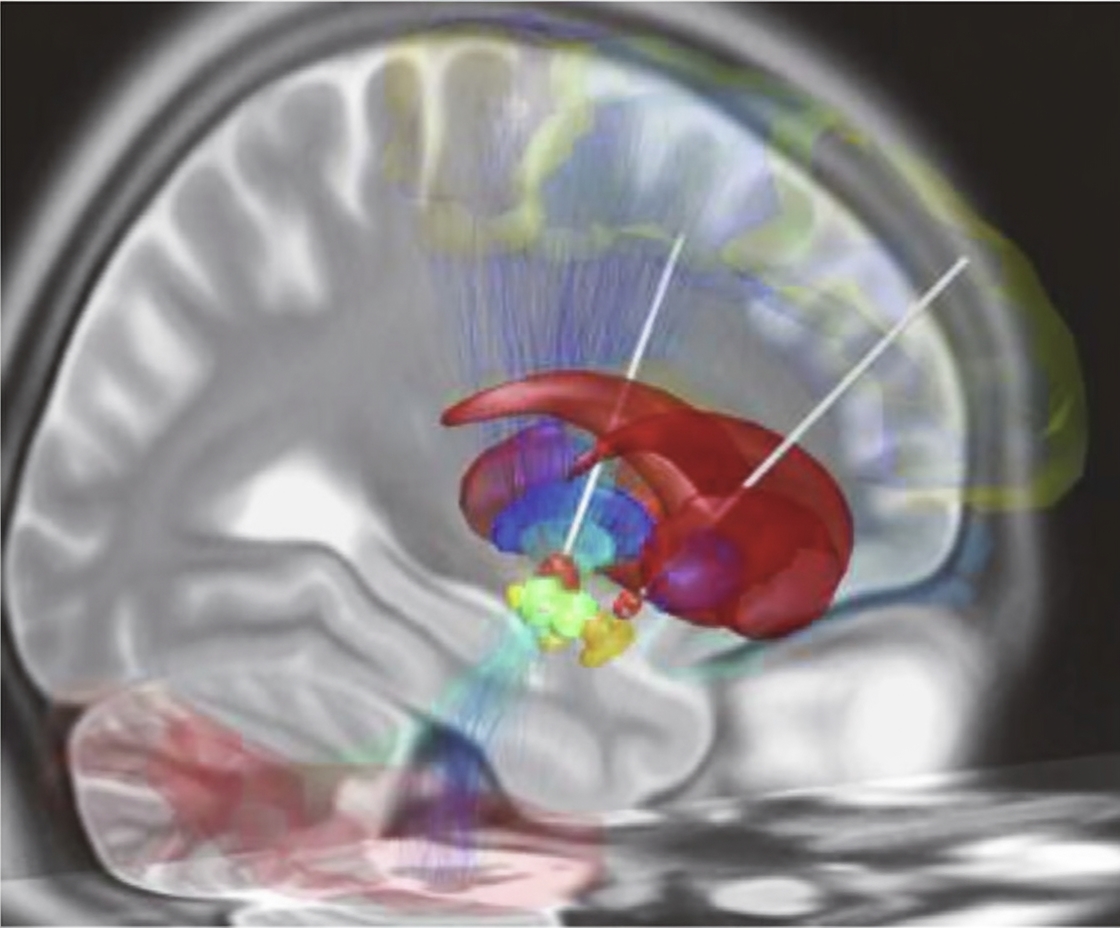
Despite demonstrated efficacy, DBS does not reach all eligible patients. Many decline invasive surgery, and trials for psychiatric uses such as treatment-resistant depression have faced setbacks. For example, a major clinical trial was stopped prematurely in 2017. Work has continued, however; recent studies have applied machine learning to identify beta-band rhythms as potential biomarkers to optimize adaptive DBS delivery. In September 2021, a UCSF team reported use of an implantable responsive neurostimulation system to treat a patient with long-standing treatment-resistant depression by detecting specific EEG patterns and delivering targeted stimulation, resulting in symptom reduction over one year.
BCIs for communication
A major research focus is enabling communication for people with aphasia or severe motor impairment through typing, handwriting decoding, or speech synthesis. In 2017 the BrainGate research team demonstrated cursor-based letter selection for three paralyzed individuals, with one participant achieving typing speeds of up to eight characters per minute. In June 2021 the BrainGate group reported a system that decoded attempted handwriting movements from the motor cortex of a tetraplegic participant, producing English text at a rate of 86 characters per minute.
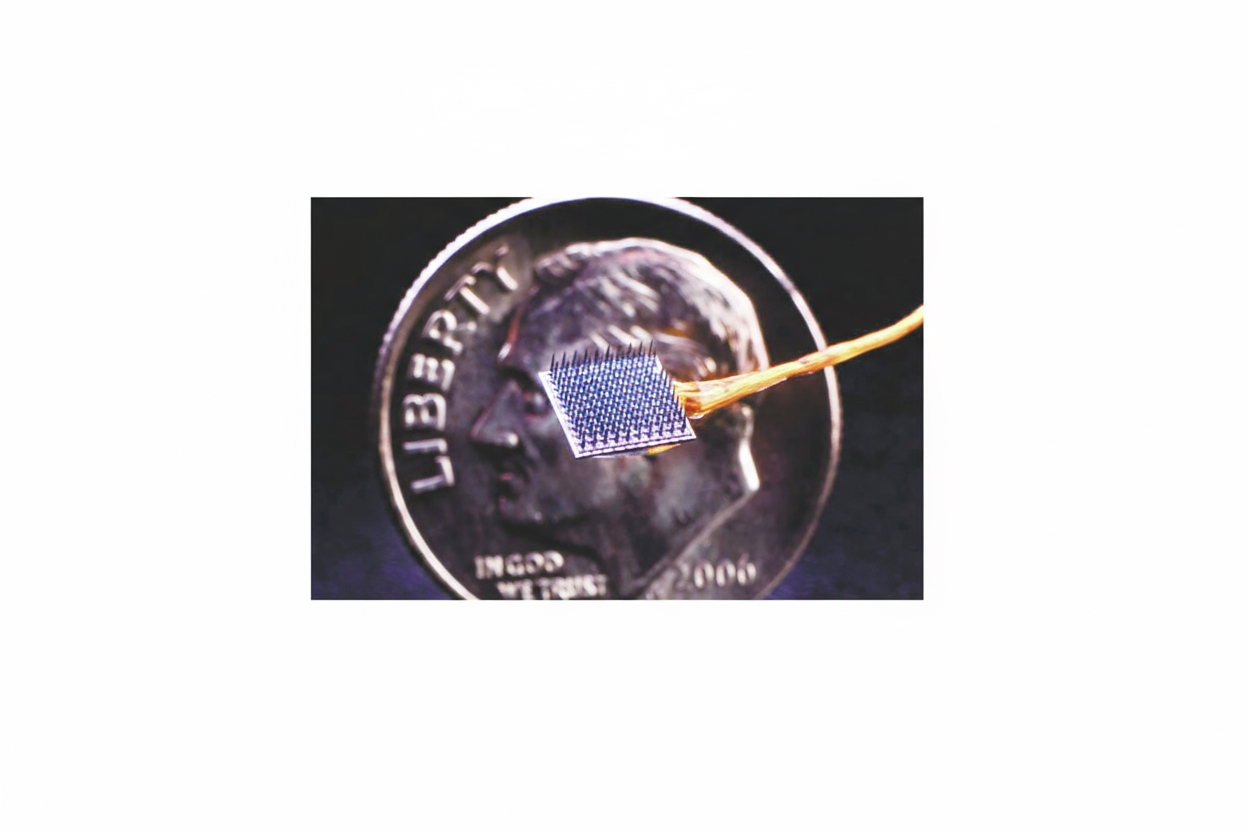
Two months later, Moses and colleagues reported progress on the system used with Pancho. Based on prior electrocorticography recordings taken during epilepsy surgery, the UCSF team implanted a rectangular array of 128 electrodes on Pancho's cortex and connected it via a fiber optic cable to a computer. Pancho attempted 50 common words such as "hungry," "music," and "computer." Custom neural network models identified fine-grained neural waveforms associated with language intent and specific words, enabling construction of a personalized language model that responded when Pancho attempted particular words. The system decoded words at a rate of roughly 15 words per minute with an accuracy of about 74% in this patient.
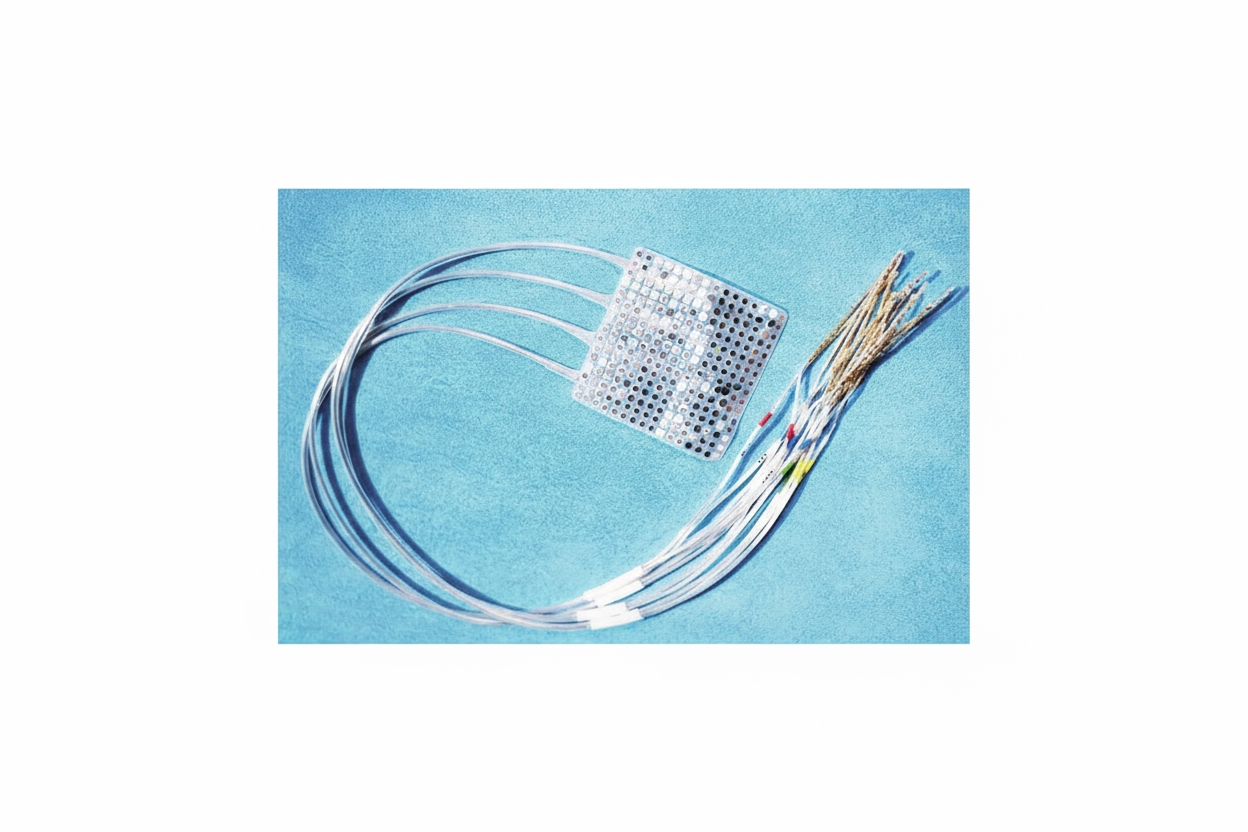
Researchers emphasize that while current vocabularies and performance are limited, the results are encouraging and likely to improve with additional effort and time. Long-term stability is also critical: continuous decoding over more than a year has been demonstrated in some studies, which is important for practical clinical use.
Industry activity and market considerations
Market analyses project substantial growth in the global BCI market over the coming decade, and several companies have invested in developing consumer-facing and clinical systems. Some industry efforts have shifted focus; for example, a technology company that previously funded research at UCSF announced a move away from invasive devices toward wrist-worn systems aimed at consumer-level neural input. Other companies, such as Neuralink, have targeted clinical applications. Neuralink's coin-sized wireless implant features over 1,000 flexible needle electrodes designed to penetrate the cortex to record and modulate neural activity. Early animal demonstrations have shown basic control of simple tasks, but extensive animal testing and regulatory review remain necessary before human trials.
Experts note that large resources can accelerate development, but technical, regulatory, and manufacturing challenges remain significant. Market factors are also critical: the high cost and currently limited market for invasive devices will constrain which technologies can be widely adopted without changes in cost structure and clinical pathways.
Challenges and outlook
Key technical challenges include long-term biocompatibility of implants, reliable signal quality, and development of robust decoding algorithms and language models that generalize across contexts. Clinical challenges include surgical risk, patient acceptance, and demonstrating meaningful functional benefit. Continued interdisciplinary progress in materials, electronics, and machine learning, combined with careful clinical study and attention to cost and scalability, will determine how widely BCIs can be used to restore communication for people with aphasia and other disorders.
 ALLPCB
ALLPCB